No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
No content results match your keyword.
Content
No product results match your keyword.
Products
Peripheral Angioplasty
Leave almost nothing behind by using Aperta NSE™ PTA scoring balloon catheter for lesion preparation in combination with SeQuent® Please OTW for drug delivery in patients with peripheral artery disease.
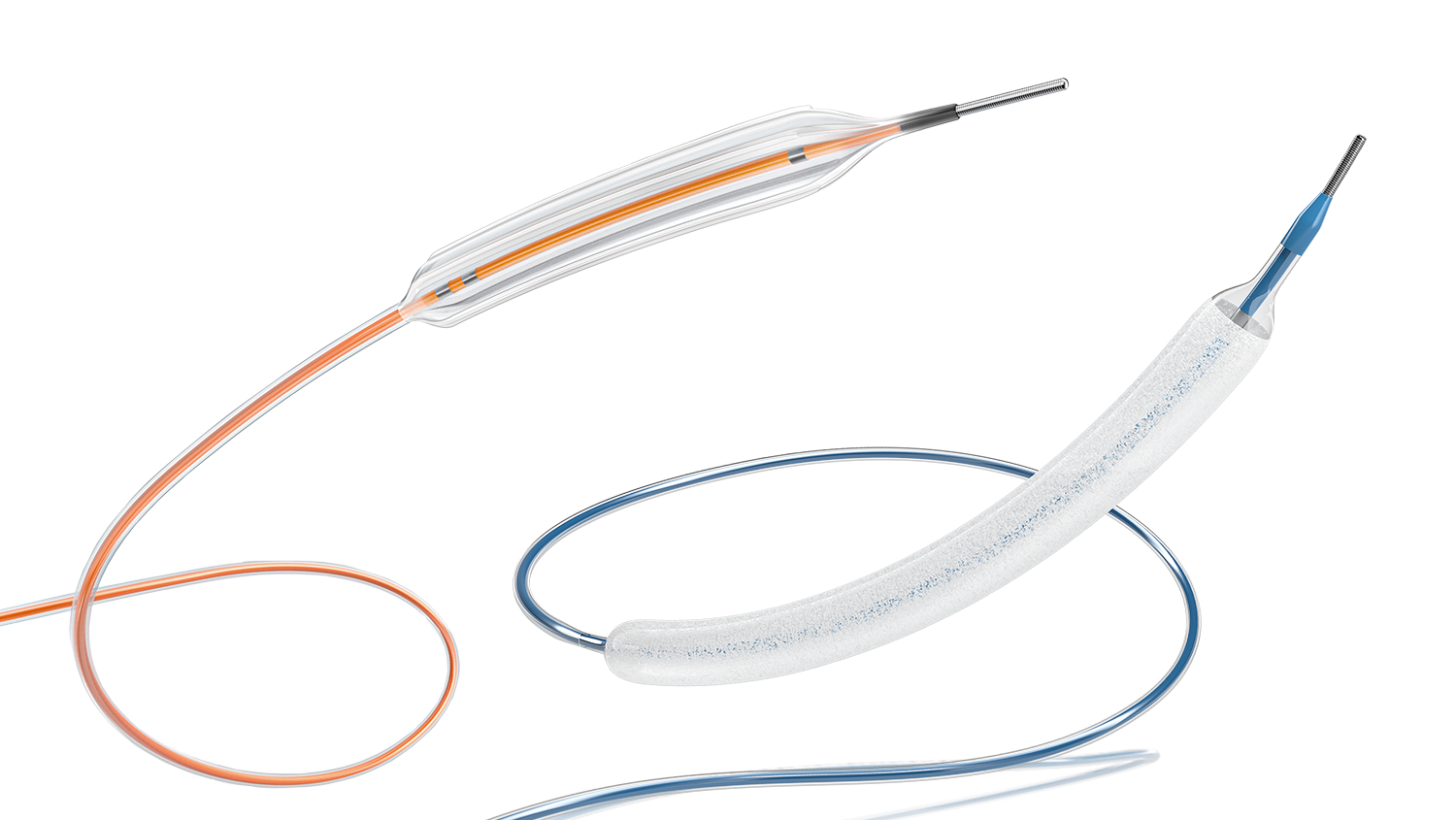
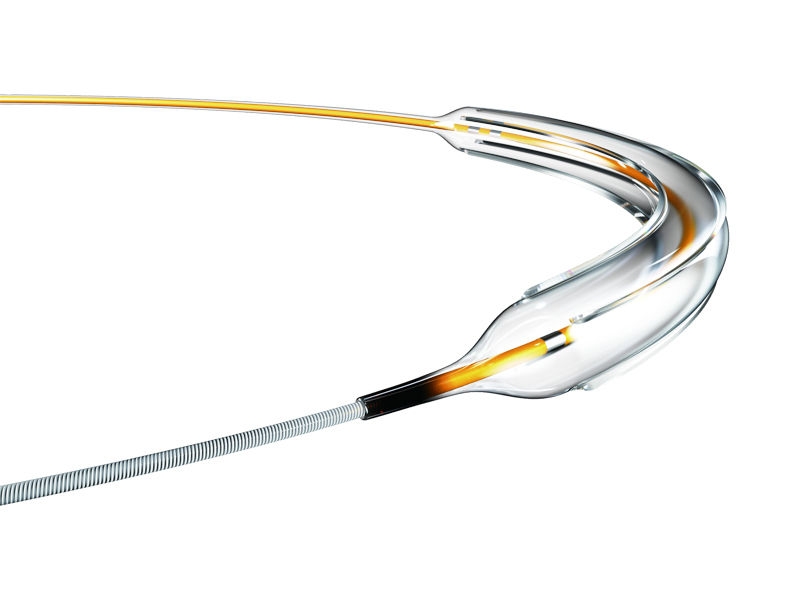
Aperta NSE™ PTA addresses the clinical needs of peripheral artery disease associated with higher rates of uncontrolled dissections, and inadequate luminal expansion. Through an innovative designed catheter, that concentrates the dilative force along four integrated scoring elements, a more predictable luminal expansion and lower rate of uncontrolled dissections can be achieved.

SeQuent® Please OTW features an optimised drug-coating technology which facilitates the safe and effective treatment of patients with peripheral arterial disease.
SeQuent® Please OTW demonstrated safety and efficacy in the CONSEQUENT randomized controlled trial (N=153) and CONSEQUENT all-comers registry (N= 784 patients) [3,4,6].