No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
No content results match your keyword.
Content
No product results match your keyword.
Products
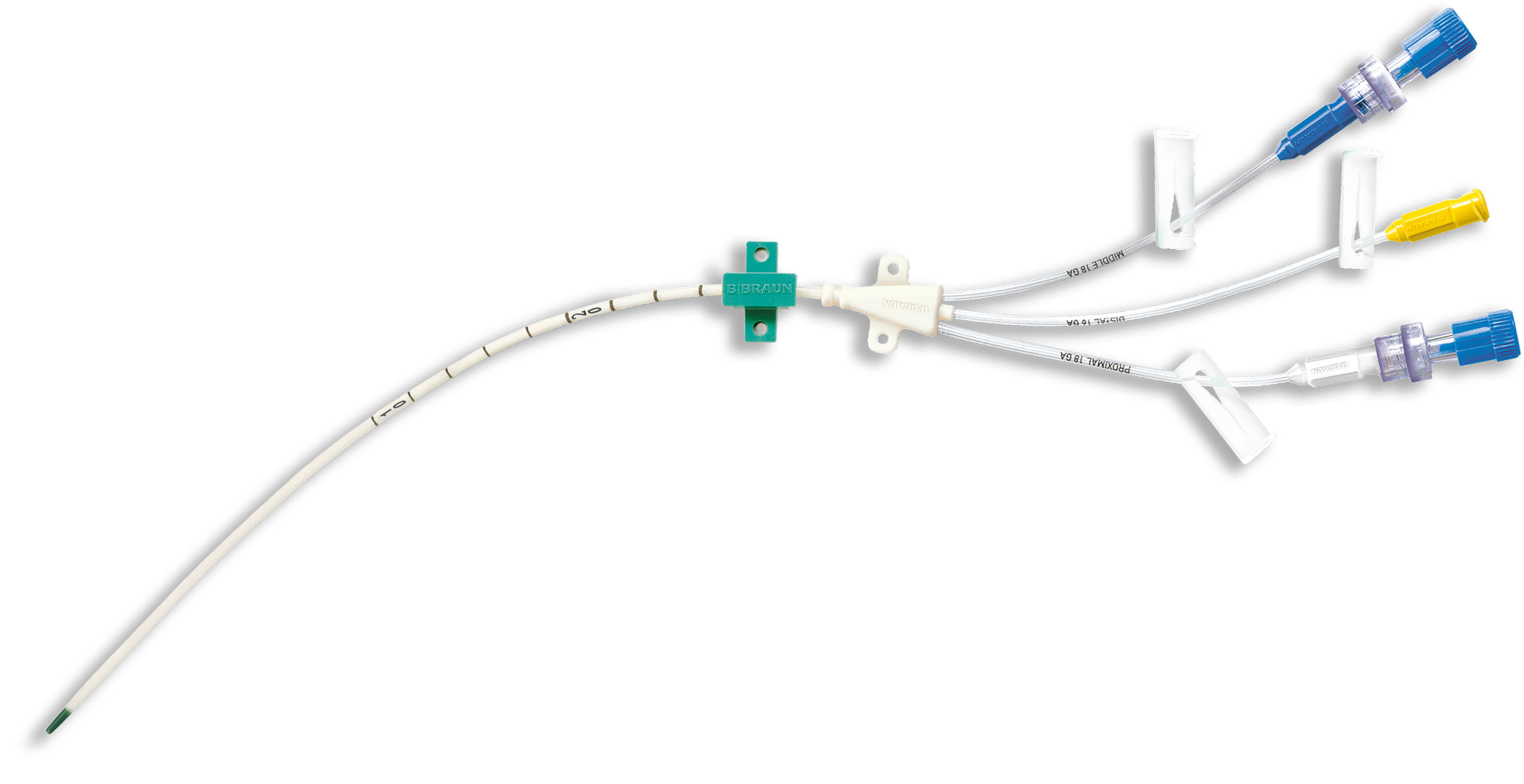
Central Venous Catheter Certofix®
B. Braun offers a comprehensive portfolio of acute central venous catheter sets for both, adult and pediatric patients.
As your system partner for ultrasound-guided central line placement and ECG-guided catheter tip positioning, our goal is to make CVC placement safer
/
To adjust optimal skin fixation position with sutured or non-sutured fixation
/
/
Additionally to the lumen configurations and the non-coated catheters, B.Braun offers a Central Venous Catheter with Proven Non-leaching Antimicrobial Effect Durable over 30 Days – Certofix® protect.
*Krikava I, et al. The efficacy of a non-leaching antibacterial central venous catheter – a prospective, randomized,
double-blind study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 Jun;164(2):154-160.

from 4,200€ to
0€
CRBSIs create additional costs per episode ranged
include a number of different components dependent on the individual set configuration, such as Introducer Needles, a Nitinol Guidewire, Syringes, Scalpels, Dilator, ECG Cable, different kind of Needle-free connectors and fixation devices. B. Braun‘s Certofix® range is complemented by a number of Accessories to confirm CVC tip position during or after placement via Intraatrial ECG. Compatible with most commonly used ECG monitors.

Active safety clip (after use) to prevent needle stick injury

Allows guidewire insertion and aspiration without disconnecting the syringe. Prevents air embolism and excessive blood loss during guidewire insertion.

The scalpel can be slid back into the housing and blocked to prevent sharps injury.

"J" and a straight tip in one wire. Both tips are flexible and suitablefor insertion. All guidewire have length markings.
Flexible ans resilient to knotting to facilitate sucessful insertion og guidewire and placement of CVC.

Certodyn® Universal Adapter and Certodyn® Universal Adapter Paed
[1] The Joint Commission. Preventing Central Line–Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources, May 2012. http://www.PreventingCLABSIs.pdf.
[2] https://ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/healthcare-associated-infections-1
[3] Yousif A, Jamal MA, Raad I. Biofilm-based central line-associated bloodstream infections. Adv Exp Med Biol. 2015; 830:157-79
[4] Elliott TSJ. The pathogenesis and prevention of intravascular catheter-related infections. In: Hamilton H, Bodenham AR. Central venous catheters. Chichester [u.a.]: Wiley-Blackwell 2009; 206-209
[5] McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012 Feb; 40(2):388-93
[6] Krikava I, et al. The efficacy of a non-leaching antibacterial central venous catheter – a prospective, randomized, double-blind study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 Jun;164(2):154-160.
[7] Richards GA, et al. Investigation of biofilm formation on a charged intravenous catheter relative to that on a similar but uncharged catheter
[8] Brunke J, Riemann T, Roschke I, 30 days antimicrobial efficacy of non-leaching central venous catheters (Poster 063), Critical Care 2016, Volume 20 Suppl 2.
[9] Brunke J, et al. Quantitative comparision of the antimicrobial efficiency of leaching versus nonleaching polymer materials. Macromol. Biosci. 2016, 16, 647-654.
[10] Hanna H, et al. Comparative in vitro efficacies and antimicrobial durabilities of novel antimicrobial central venous catheters. Antimicrob Agents Chemother. 2006 Oct;50(10):3283-8
[11] Yasukawa T, Fujita Y, Sari A. Antimicrobialimpregnated central venous catheters. N Engl J Med. 1999 Jun 3; 340(22):1762
[12] Oda T, Hamasaki J, Kanda N, Mikami K. Anaphylactic shock induced by an antiseptic-coated central venous catheter. Anesthesiology. 1997 Nov; 87(5):1242-4.
[13] Tambe SM, Sampath L, Modak SM. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemother 2001; 47: 589-98
[14] Sampath LA, Tambe SM, Modak SM. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect Control Hosp Epidemiol 2001; 22: 640-6