No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
No content results match your keyword.
Content
No product results match your keyword.
Products
Treatment of hydrocephalus
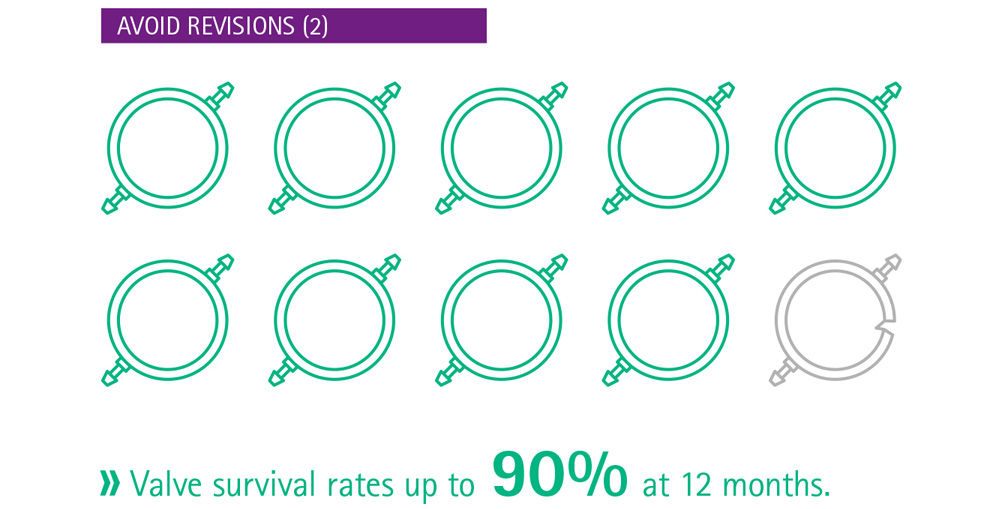
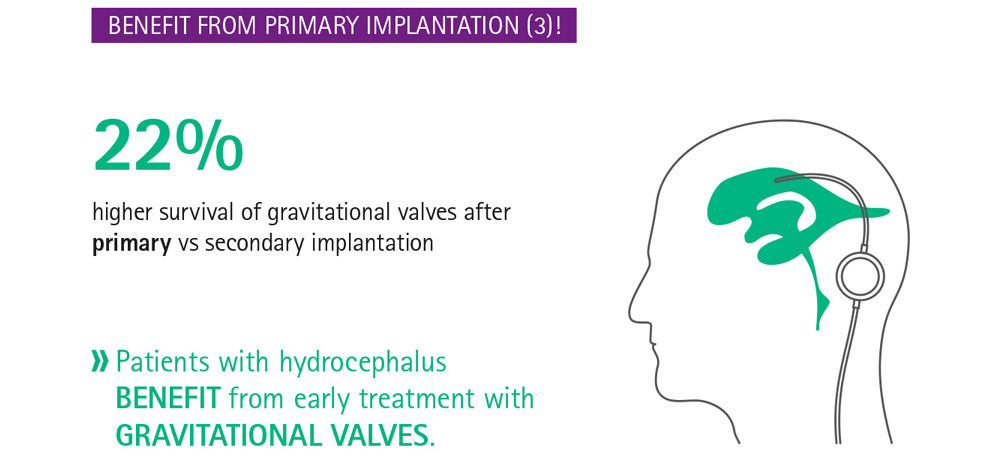
The critical issue in shunt technology is the posture-dependent hydrostatic pressure change.
Current evidence suggests that delay in treatment is harmful for the patient, [7] even when the delay is as short as 3 months. [8] Especially in infants with hydrocephalus, whose brains are still developing, the primary treatment choice and possible complications have a relevant impact on the long-term outcome. [9] Infants who received a gravitational shunt as part of a shunt revision had a lower rate of shunt survival than those with a primary implantation of a gravitational shunt, [9] stressing the importance of optimal primary implantation. Delay in treatment of hydrocephalus may be harmful and early intervention is indicated, particularly when considering that not all complications are reversible. Hence, it is important to make use of the best available treatment and to get it right the first time.
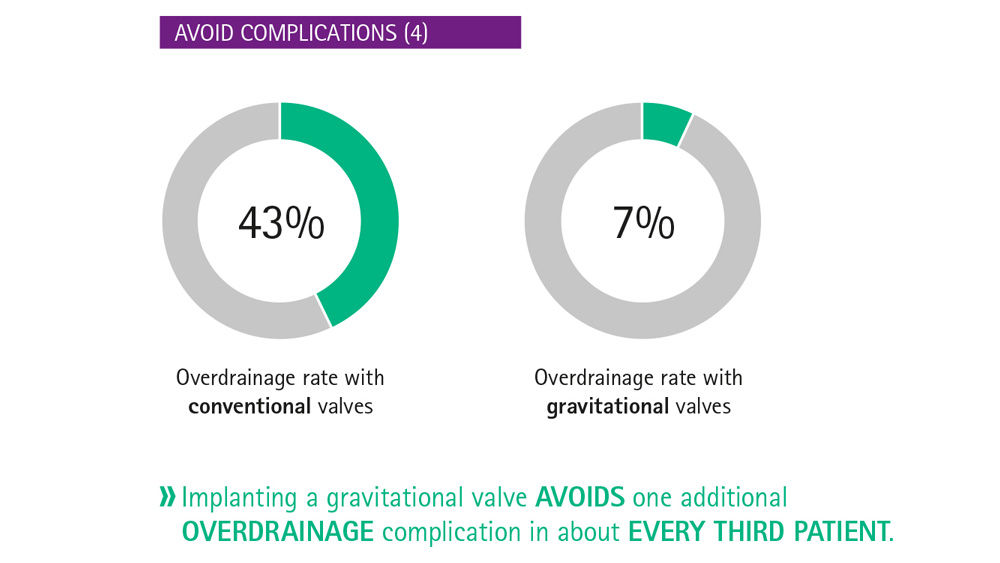
The critical issue in shunt technology is to simulate the posture-dependent hydrostatic pressure change. [10] Gravitational shunts, which compensate for posture-dependent hydrostatic pressure changes, can prevent overdrainage. [11] In the horizontal position, the gravitational valve opens completely and hence does not contribute to the overall flow resistance, while in the vertical position, the flow resistance is determined by both the gravitational valve and the differential pressure valve.
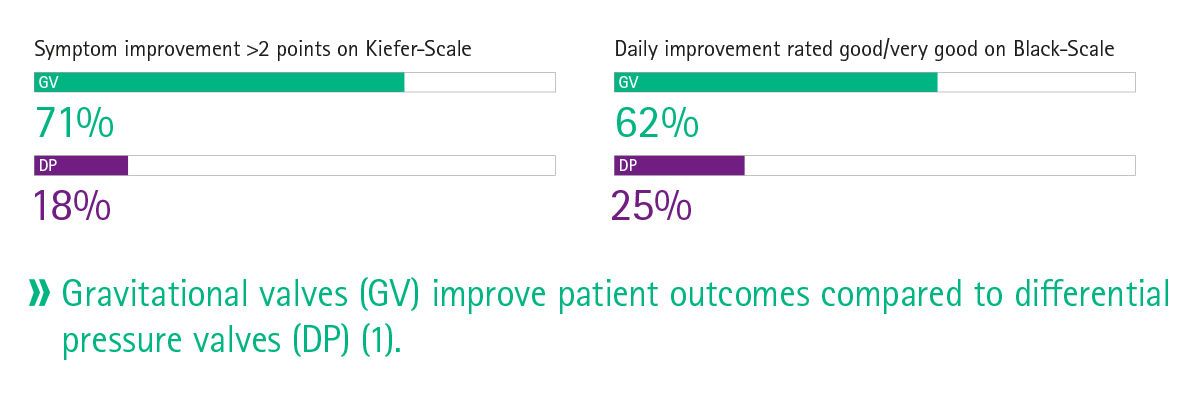
Clinical practice has shown that the usage of programmable valves with MIETHKE gravitational shunts can lower the number of shunt revisions due to over- and underdrainage, [3] improve symptoms and the overall daily functioning. [10] Further, gravitational shunts have been shown to be efficacious in the treatment of hydrocephalus in infants. [12]
MIETHKE gravitational shunts can compensate for overdrainage without compromising the setting for the lying position. The optimal opening pressure for each patient when standing and when lying can be set – without needing to compromise.
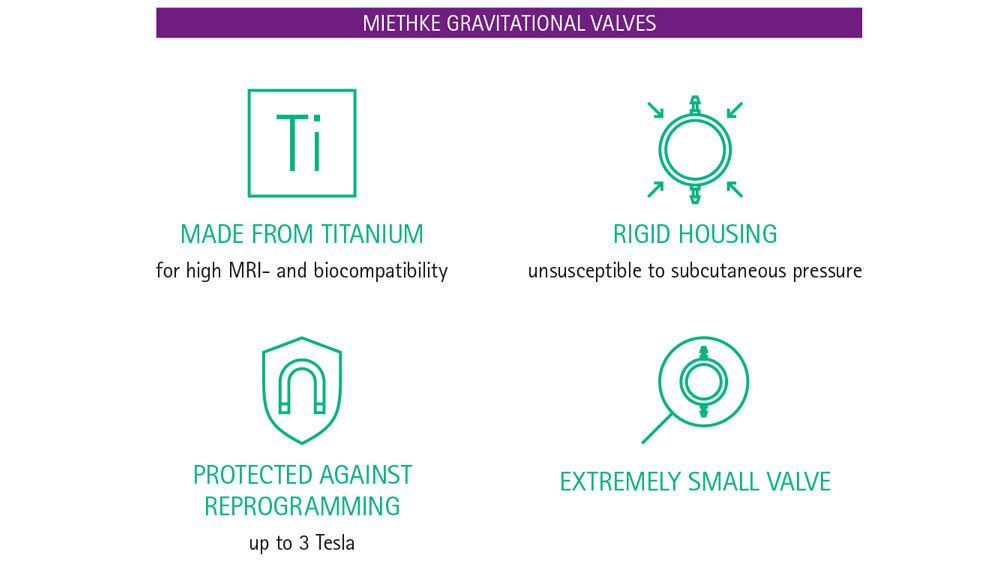
The unintentional reprogramming of valves by low-intensity magnetic fields is of great concern for patients and physicians alike. But how can one be sure that a valve will not be reprogrammed accidentally? The key question surely is whether the programmable valve is protected from reprogramming by the strong fields generated during MRI. If the magnetic fields of an MR system cannot reset the setting then low-intensity magnetic fields should also have no influence. Programmable MIETHKE valves have an ‘Active-Lock mechanism’, which protects them from reprogramming by magnetic fields of up to 3 Tesla. [13]
When developing a hydrocephalus valve which offers an optimal individual treatment, it is crucial to focus on the flexible adjustability of the opening pressure during the active time of the day, i.e., the upright body position. After fixed pressure gravitational valves and adjustable gravitational valves, the M.blue® provides neurosurgeons and patients with the next generation of hydrocephalus valves operating in a position-dependent manner. M.blue® is the essence of 26 years of experience with valve technology for hydrocephalus and the feedback of numerous doctors and patients worldwide.
[1] Tschan CA, Antes S, Huthmann A, et al. Overcoming CSF overdrainage with the adjustable gravitational valve proSA. Acta Neurochir (Wien) 2014;156(4):767-76; discussion 76.
[2] Sprung C, Schlosser HG, Lemcke J, et al. The adjustable proGAV shunt: a prospective safety and reliability multicenter study. Neurosurgery 2010;66(3):465-74.
[3] Gebert AF, Schulz M, Schwarz K, Thomale UW. Long-term survival rates of gravity-assisted, adjustable differential pressure valves in infants with hydrocephalus. J Neurosurg Pediatr 2016 17:544-551
[4] Lemcke J, Meier U, Müller C, Fritsch M, Kehler U, Langer N et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial (SVASONA). J Neurol Neurosurg Psychiatry 2013 84(8):850-857
[5] Thomale UW, Gebert AF, Haberl H, et al. Shunt survival rates by using the adjustable differential pressure valve combined with a gravitational unit (proGAV) in pediatric neurosurgery. Childs Nerv Syst 2013;29(3):425-31.
[6] Lemcke J, Meier U, Muller C, et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial (SVASONA). J Neurol Neurosurg Psychiatry 2013;84(8):850-7.
[7] Sidambe AT. Biocompatibility of Advanced Manufactured Titanium Implants-A Review. Materials (Basel, Switzerland) 2014;7(12):8168-88.
[8] Saini M, Singh Y, Arora O, et al. Implant biomaterials: A comprehensive review. World journal of clinical cases 2015;3(1):52-7.
[9] Toma AK, Watkins LD. Surgical management of idiopathic normal pressure hydrocephalus: a trial of a trial. Br J Neurosurg 2016;30(6):605.
[10] Toma AK, Stapleton S, Papadopoulos MC, et al. Natural history of idiopathic normal-pressure hydrocephalus. Neurosurg Rev 2011;34(4):433-9.
[11] Irving G, Neves AL, Dambha-Miller H, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 2017;7(10):e017902.
[12] Suchorska B, Kunz M, Schniepp R, et al. Optimized surgical treatment for normal pressure hydrocephalus: comparison between gravitational and differential pressure valves. Acta Neurochir (Wien) 2015;157(4):703-9.
[13] Chari A, Czosnyka M, Richards HK, Pickard JD, Czosnyka ZH. Hydrocephalus shunt technology: 20 years of experience from the Cambridge Shunt Evaluation Laboratory, J Neurosurg 2014;120(3): 697-707.