No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
MIETHKE M.blue®

The M.blue® is a position-dependent hydrocephalus valve. It consists of an adjustable gravitational unit and a fixed differential pressure unit. Particularly challenging forms of hydrocephalus require a much higher degree of flexibility in treatment. This is what the M.blue® plus stands for: a combination of adjustable gravitational unit and adjustable differential pressure unit (proGAV® 2.0).

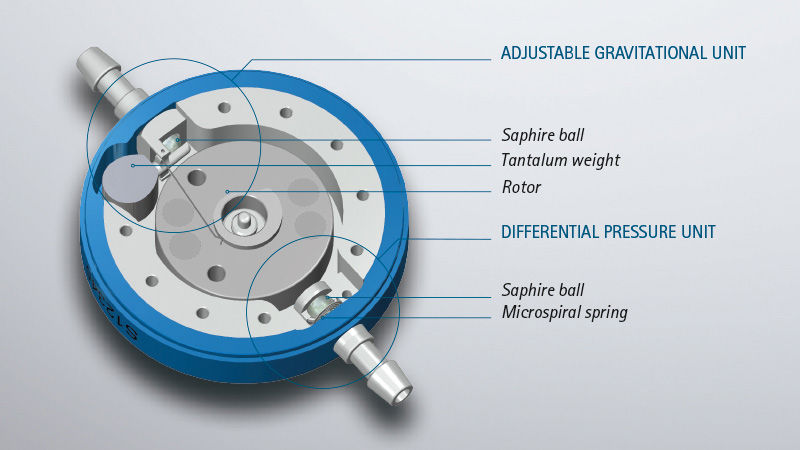
The combination of these two units ensures automatic adjustment of the opening pressure depending on the patient's body position and thus counteracts the risk of possible overdrainage complications, especially in the upright and active body position. [5], [6], [7], [8] Particularly challenging forms of hydrocephalus require a much greater flexibility in treatment. This is what the M.blue plus® stands for: a combination of an adjustable gravitational unit and an adjustable differential pressure unit (proGAV® 2.0). Both M.blue® variants are made of robust and biocompatible titanium and are the result of precise precision engineering.
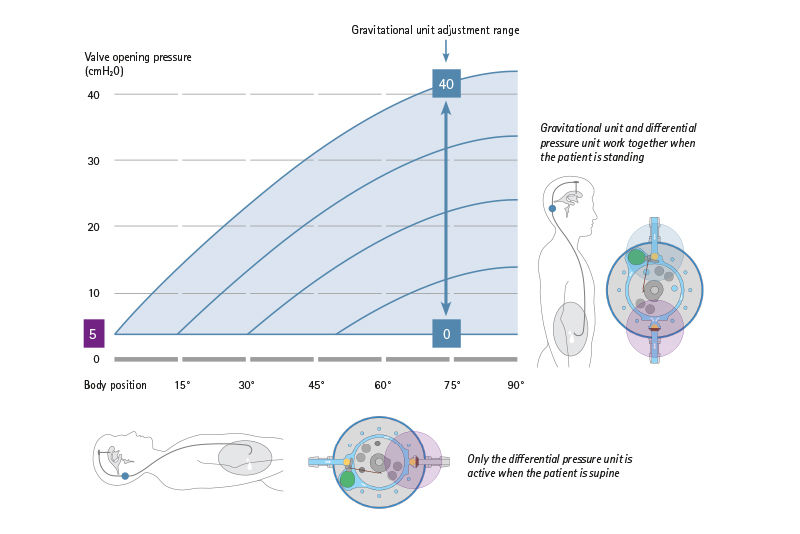
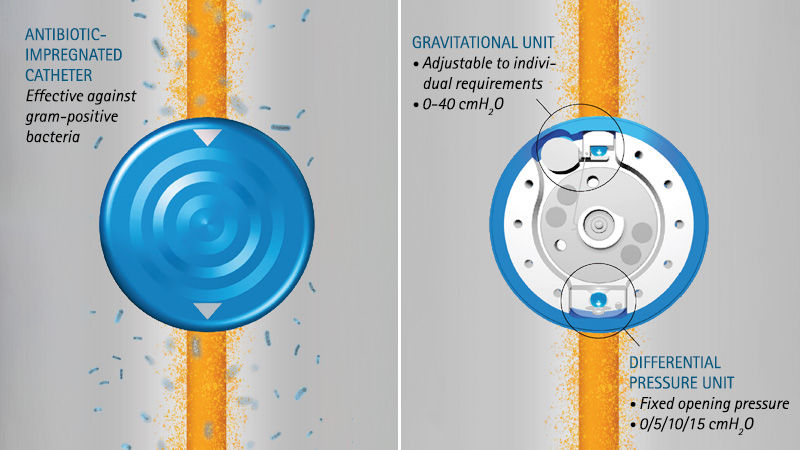
The differential pressure unit in the M.blue® is available in the units 0, 5, 10 and 15 cmH2O. The selected opening pressure of the differential pressure unit is equally effective in all body positions. When lying down, the differential pressure unit acts alone and usually has a low selected opening pressure, so that the valve drains brain water in time when the brain pressure increases during sleep and rest phases. A microspiral spring that presses a sapphire ball into a ball seat is responsible for the correct pressure. If the brain pressure rises above the selected opening pressure, the spring gives way, the differential pressure valve opens and allows brain fluid to pass through until the brain pressure has dropped again accordingly.
The gravitational unit in the M.blue® is adjustable from 0-40 cmH2O and is only effective in the upright body position. Now the sum of both units – gravitational and differential pressure unit – determines the total opening pressure of the M.blue®.
More information about the different pressure levels and their X-ray detection can be found in the download area below.

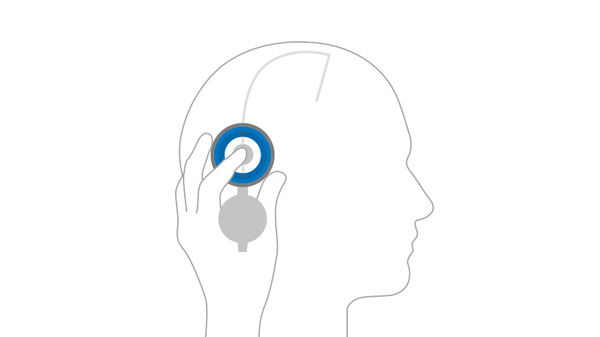
Locate valve by palpating the area with your finger through the open M.blue plus® compass.
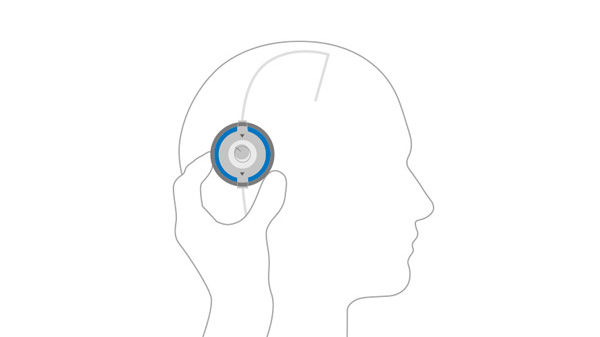
Close M.blue plus® compass and use the floater to lock location and read current valve opening pressure setting.
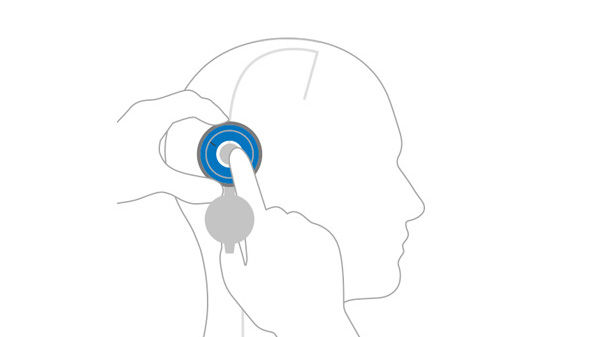
With the help of the inserted adjustment ring, the valve opening pressure can easily be set to the desired level. After setting the valve opening pressure, it is advisable to double-check the pressure level settings.

One of the most common and potentially serious complications of hydrocephalus treatment is an infection of the shunt, [13] affecting 7-15% of hydrocephalus patients. [14] Shunt infections can have severe consequences for the patient. [15-18]
Use of antibiotic-impregnated catheters can prevent two-thirds of shunt infections, [19] thus helping to reduce patient burden and improve patient outcome.
MIETHKE’s new antibiotic-impregnated catheter XABO® uses a balanced ratio to effectively fight gram-positive bacteria. [20]
XABO® antibiotic impregnated catheters perfectly connect to our innovative MIETHKE M.blue® valve. It offers long-lasting antimicrobial effects for at least 38 days after implantation, easy handling as it withstands temperatures up to 30°C without losing its effectiveness [21] and convenient storage for up to 63 months.
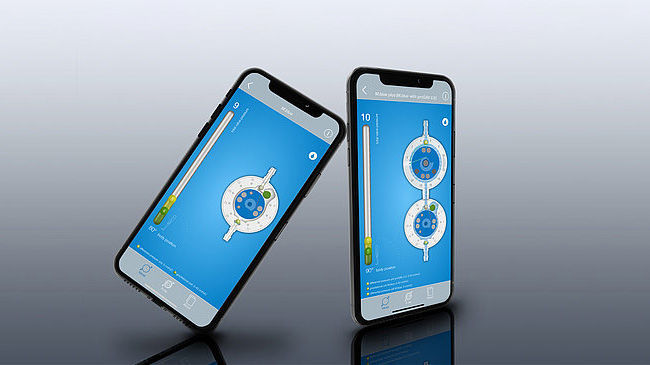
Functionality of the adjustment and the position-dependent opening pressures are shown clearly on the MIETHKE App, available on Android or iOS.
iTunes preview and download >>
Google Play preview and download >>
[1] 2010, Mar, Sprung C., Schlosser H. G., Lemcke J., Meier U., Messing-Junger M., Trost H. A., Weber F., Schul C., Rohde V., Ludwig H. C., Hopfner J., Sepehrnia A., Mirzayan M. J. and Krauss J. K.: "The adjustable proGAV shunt: a prospective safety and reliability multicenter study", Neurosurgery 66(3): 465-474
[2] 2015, Oct, Kehler U., Kiefer M., Eymann R., Wagner W., Tschan C. A., Langer N., Rohde V., Ludwig H. C., Gliemroth J., Meier U., Lemcke J., Thomale U. W., Fritsch M., Krauss J. K., Mirzayan M. J., Schuhmann M. and Huthmann A.: "PROSAIKA: a prospective multicenter registry with the first programmable gravitational device for hydrocephalus shunting", Clin Neurol Neurosurg 137: 132-136
[3] 2018, Jan, Antes S., Stadie A., Muller S., Linsler S., Breuskin D. and Oertel J.: "Intracranial Pressure-Guided Shunt Valve Adjustments with the Miethke Sensor Reservoir", World Neurosurg 109: e642-e650
[4] NPH-Leitlinien der DGN 2018
[5] 2013, Lemcke J., Meier U., Muller C., Fritsch M. J., Kehler U., Langer N., Kiefer M., Eymann R., Schuhmann M. U., Speil A., Weber F., Remenez V., Rohde V., Ludwig H. C. and Stengel D.: "Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial (SVASONA)", J Neurol Neurosurg Psychiatry 84(8): 850-857
[6] 2014, Tschan C. A., Antes S., Huthmann A., Vulcu S., Oertel J. and Wagner W.: "Overcoming CSF overdrainage with the adjustable gravitational valve proSA", Acta Neurochir (Wien) 156(4): 767-776,
[7] 2017, May, Alavi S.,Schulz M., Schaumann A., Schwarz K. and Thomale U. W.: "Valve exchange towards an adjustable differential pressure valve with gravitational unit, clinical outcome of a single-center study", Childs Nerv Syst 33(5): 759-765
[8] 2016, May, Gebert A. F., Schulz M., Schwarz K. and Thomale U. W.: "Longterm survival rates of gravityassisted, adjustable differential pressure valves in infants with hydrocephalus", J Neurosurg Pediatr 17(5): 544-551
[9] 2016, May 15, Miyake H.: "Shunt Devices for the Treatment of Adult Hydrocephalus: Recent Progress and Characteristics", Neurol Med Chir (Tokyo)
[10] 2017, Pierson M. J., Wehrmann D., Albers J. A., El Tecle N. E., Costa D. and Elbabaa S. K.: "Programmable shunt valve interactions with osseointegrated hearing devices", J Neurosurg Pediatr 19(4): 384-3
[11] 2019, Aschoff A: "In-Depth View: Functional Characteristics of CSF Shunt Devices (Pros and Cons)", Textbook of Pediatric Neurosurgery, Editor: C. Di Rocco90 56(5): 274-28210
[12] 2020, Gutowski P., Golz L., Rot S., Lemcke J. and Thomale U. W.:"Gravitational shunt valves in hydrocephalus to challenge the sequelae of over-drainage", Expert Rev Med Devices 17(11): 1155-1168
[13] Okamura Y, Maruyama K, Fukuda S, et al. Detailed standardized protocol to prevent cerebrospinal fluid shunt infection. J Neurosurg 2019:1-5.
[14] Fernández-Méndez R, Richards HK, Seeley HM, et al. Current epidemiology of cerebrospinal fluid shunt surgery in the UK and Ireland (2004-2013). J Neurol Neurosurg Psychiatry 2019;90(7):747-54.
[15] Blount JP, Campbell JA, Haines SJ. Complications in Ventricular Cerebrospinal Fluid Shunting. Neurosurgery Clinics of North America 1993;4(4):633-56.
[16] Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004;350(14):1422-29.
[17] Walters BC, Hoffman HJ, Hendrick EB, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 1984;60(5):1014-21.
[18] Sciubba DM, Stuart RM, McGirt MJ, et al. Effect of antibiotic-impregnated shunt catheters.
[19] Mallucci CL, Jenkinson MD, Conroy EJ, et al. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. The Lancet 2019;394(10208):1530-39.
[20] MIETHKE report. Data on file.
[21] MIETHKE report. Data on file.
[22] 2013, Nov, Gebert A. F., Schulz M., Haberl H. and Thomale U. W.: "Adjustments in gravitational valves for the treatment of childhood hydrocephalus-a retrospective survey", Childs Nerv Syst 29(11): 2019-2025