No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
Unfold new spaces for fusion
Inspired by human anatomy, empowered by science – our cages fuse technological advancements with clinical values. The result is a major leap in anterior and posterior stabilisation.
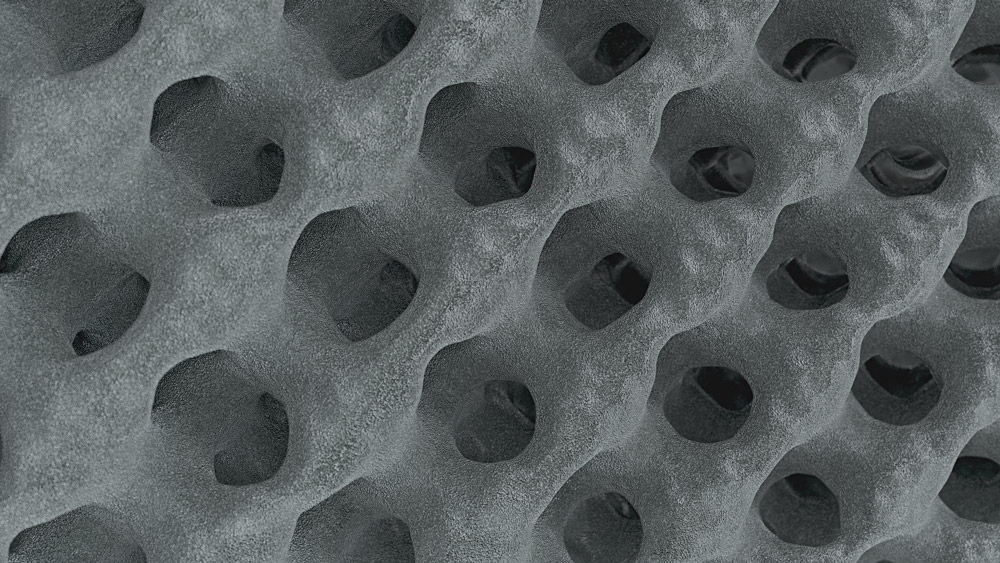
Structan®
You think this is an ordinary cage lattice? Prepare to be impressed by the science behind Structan®. Decades of experience, combined with modern technology, have led to the creation of it – a structure designed for improved clinical outcomes and advanced biomechanical performance.
Surface area is magnified by
0
times, providing more opportunities for bone ingrowth.
Strong and elastic at the same time – Structan® is
0%
closer to the elastic modulus of cortical bone. (1-4) *
Cover posterior stabilisation with just
0
modular spinal platform that adapts precisely to your needs.

The finely balanced surface roughness has a positive influence on the adhesion of osteoblasts. The porosity aligns with the human anatomy. This creates a solid basis for bony on-growth and thus fusion with Structan®.[5-8]
/
Substantial osteoblastic differentiation and improved osseointegration – based on scientific evidence, our AESCULAP® 3D Cages reflect the biological attributes of the trabecular structure, empowering bony in-growth.[9-15]
/
Smart designed graft window to support osseointegration between bone and implant – with or without autograft or allograft.
/
The harmonized interface provides a firm connection to instruments and high precision during handling. Feel all of this with our articulating inserter in TLIF procedures – because confidence delivers peace of mind.
/
AESCULAP® 3D interbody fusion devices
As with the creation of all of our spine solutions, the design of the AESCULAP® 3D interbody fusion devices is based on our core values of creating advanced biomechanical performance, intra-operative flexibility and improved clinical outcomes.
Additive manufacturing
Structan®
The portfolio
Surgical workflow animations
Take a look at the performance of AESCULAP® 3D interbody fusion devices and Ennovate®.
Our TLIF interbody fusion device, with its articulating inserter, enables true minimally invasive fusion procedures.
With its streamlined surgical technique, our PLIF interbody fusion device and Ennovate® are ideal for the open approach.
Merging the essence of two worlds – our TLIF interbody fusion device can be implanted in a minimally invasive and an open approach.
Discover the AESCULAP® spinal platform
*compared to solid titanium alloy interbody fusion devices.
[1] Kuhn JL, Goldstein SA, Choi K, London M, Feldkamp LA, Matthews LS. Comparison of the trabecular and cortical tissue moduli from human iliac crests. J Orthop Res. 1989;7(6):876 84.
[2] Ratner BD, Hoffmann AS, Schoen FJ, Lemons JE. An Introduction to Materials in Medicine. Academic Press. 1996.
[3] Chen Y, Wang X, Lu X, Yang L, Yang H, Yuan W, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7 year follow up. Eur Spine J. 2013;22(7):1539 46.
[4] Brizuela A, et al. Influence of the elastic modulus on the Osseointegration of Dental Implants. Materials. 2019;12(6):980.
[5] Bostrom M, Boskey A, Kaufman J, Einhorn T. Form and function of bone. In: Orthopaedic Basic Science Biology and Mechanics of the Musculoskeletal System. 2nd ed. Rosemont, IL: AAOS; 2000: 320-369.
[6] Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J 2012; 12:265-272.
[7] Lincks, J. et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 19, 1998. Pages 2219-32.
[8] Elias CN, et al. Mechanical and clinical properties of titanium and titanium based alloys ( Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. Journal of Materials Research and Technology. 2019;8(1):1060 9.
[9] Cheng A, Cohen D, Boyan B et al. Laser-Sintered Constructs with Bio-inspired Porosity and Surface Micro/ Nano-Roughness Enhance Mesenchymal Stem Cell Differentiation and Matrix Mineralization In Vitro. Calcif Tissue Int 2016; 99:625–637.
[10] Wu S-H, Li Y, Zang Y-Q, et al. Porous Titanium-6 Aluminum-4 Vanadium Cage Has Better Osseointegration and Less Micromotion Than a Poly-Ether-Ether-Ketone Cage in Sheep Vertebral Fusion. Art Organs 2013; 37:191-201
[11] Taniguchi N, Fujibayashi S, Takemoto M, Sasaki K, Otsuki B, Nakamura T, Matsushita T, Kokubo T, Matsuda S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Materials Science and Engineering 2016; C59: 690–701.
[12] Changhui Song, Lisha Liu, Zhengtai Deng, Haoyang Lei, Fuzhen Yuan, Yongqiang Yang, Yueyue Li, Jiakuo Yu. Research progress on the design and performance of porous titanium alloy bone implants. Journal of Materials Research and Technology, Volume 23, 2023. Pages 2626-2641, ISSN 2238-7854.
[13] Fukuda A, Takemoto M, Saito T, et al. Osteoinduction of porous Ti implants with a channel structure fabricated by Selective Laser Melting. Acta Biomat 2011; 7:2327-2336.
[14] Ran Q, Yang W, Hu Y, Shen X, Yu Y, Xiang Y, Cai K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J Mech Behav Biomed Mater. 2018 Aug;84:1-11. doi: 10.1016/j.jmbbm.2018.04.010. Epub 2018 Apr 18. PMID: 29709846.
[15] Van Bael S, Chai YC, Truscello S, Moesen M, Kerckhofs G, Van Oosterwyck H, Kruth JP, Schrooten J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012 Jul;8(7):2824-34. doi: 10.1016/j.actbio.2012.04.001. Epub 2012 Apr 7. PMID: 22487930.
[16] Kia, C.; Antonacci, C.L.; Wellington, I.; Makanji, H.S.; Esmende, S.M. Spinal Implant Osseointegration and the Role of 3D Printing: An Analysis and Review of the Literature. Bioengineering 2022, 9, 108. https://doi.org/10.3390/bioengineering9030108.
[17] Usability-Test, Usability Validation of AESCULAP® CeSPACE® 3D Cages, Tübingen, 2019.The usability of the AESCULAP® 3D Cage System CeSPACE® 3D was tested in April 2019, in a cadaver workshop with six independent test persons as intended users (surgeons specialized in spinal surgery or comparable fields). Parameters such as implant visibility under x-ray control, mechanical stability of the implant/instrument interface andimplant surface evaluation in terms of tissue injury risk were tested among others. Acceptance criteria were fulfilled for all the above-mentioned parameters. All test users confirmed the absence of critical features that must be improved prior to clinical use. During the test, the x-ray visibility of the cages was particularly positively assessed.
[18] Rehnitz, Christoph, PD Dr. med. Radiological image evaluation of AESCULAP® interbody fusion devices, Heidelberg, 2019. CT and X-ray visualization of different AESCULAP® interbody fusion cages (full titanium, porous Ti6Al4V and PLASMAPOREXP® cages) was tested in a cadaver setup. A radiologist evaluated the implant visibility and the presence of artefacts that may limit the visualization of adjacent structures. Visualization and assessment of implant position was achieved in X-ray and CT for all tested cages. Minor artefacts were visible in CT reconstructions in the surrounding of porous Ti6Al4V and full titanium implants. Porous Ti6Al4V implants showed slightly fewer artefacts in CT in comparison to full titanium implants. The minor artefacts observed did not limit the assessment of the surrounding anatomical structures.